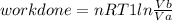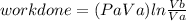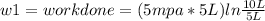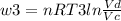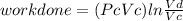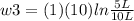Physics, 19.02.2021 17:00 bkimswift7
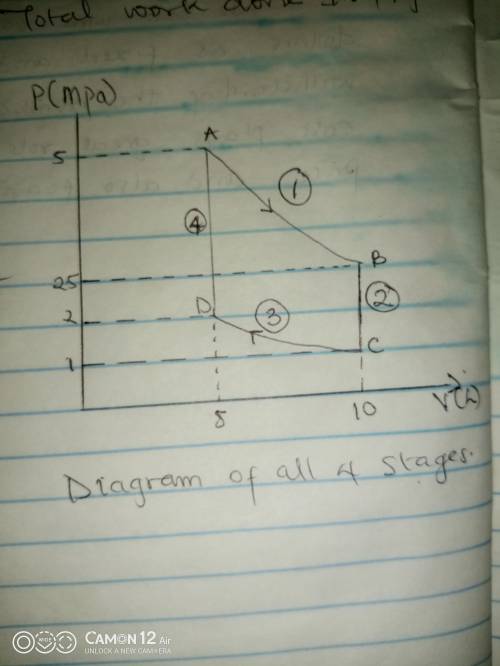
Thermodynamic Processes Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally until the volume is doubled (step 1). Then it is cooled isochorically until the pressure is 1 MPa (step 2). The temperature drops in this process. The gas is now compressed isothermally until its volume is back to 5 L, but its pressure is now 2 MPa (step 3). Finally, the gas is heated isochorically to return to the initial state (step 4). (a) Draw the four processes in the pV plane. (b) Find the total work done by the gas.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 10:30, elijahjacksonrp6z2o7
The freezing and boiling point of a substance changes as the air pressure around it changes. for example, at a lower air pressure (higher altitude) it is easier for water molecules to escape from liquid into the air. in a high altitude city such as denver, colorado compared to a sea-level city such as houston, texas, water
Answers: 2


Physics, 22.06.2019 15:50, potatocow
The california mussel (mytilus californianus) attaches itself to a rock or other solid surface with a bundle of filaments known as the byssus. imagine that 15.0 j of work is done to stretch the distal end of the byssus. it releases 10.8 j of thermal energy as it relaxes. what is the resilience of the distal end of the byssus?
Answers: 2
You know the right answer?
Thermodynamic Processes
Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally...
Questions in other subjects:


Biology, 27.01.2021 07:50


Mathematics, 27.01.2021 07:50



Social Studies, 27.01.2021 07:50

Health, 27.01.2021 07:50

Mathematics, 27.01.2021 07:50