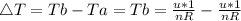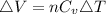Consider a system consisting of an ideal gas confined within a container, one wall of which is a movable piston. Energy can be added to the gas in the form of heat by applying a flame to the outside of the container. Conversely, energy can also be removed from the gas in the form of heat by immersing the container in ice water. Energy can be added to the system in the form of work by pushing the piston in, thereby compressing the gas. Conversely, if the gas pushes the piston out, thereby pushing some atmosphere aside, the internal energy of the gas is reduced by the amount of work done.

Answers: 3


Other questions on the subject: Physics


Physics, 22.06.2019 14:50, lilyrockstarmag
Abar magnet cut in half will form a total ofpoles. a)fourb)eightc)two
Answers: 1

Physics, 22.06.2019 16:00, chamyaparker
What is the freezing point of radiator fluid that is 50% antifreeze by mass? kf for water is 1.86 ∘c/m.
Answers: 3

Physics, 22.06.2019 17:00, deathray4035
Photons with the highest energy have the longest period
Answers: 1
You know the right answer?
Consider a system consisting of an ideal gas confined within a container, one wall of which is a mov...
Questions in other subjects:










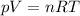
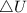 as the system of ideal gas goes from point A to point B on the graph recall u is proportional to T
as the system of ideal gas goes from point A to point B on the graph recall u is proportional to T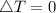
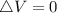
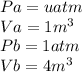
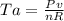
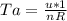
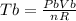
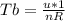
 is mathematically given as
is mathematically given as