
Physics, 29.01.2021 06:40 gustavoroggero39
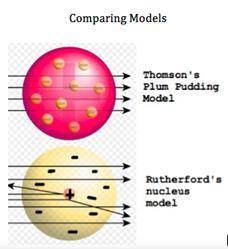
Atomic models have changed over the decades. Two early atomic models can be seen here. There is a dramatic change in the models, as Rutherford experimented with the cathode ray tube and charged particles. Differentiate between the two models by selecting the main difference between the models.
Question 5 options:
Rutherford's model shows negative charges dispersed throughout the atom
Rutherford's model shows negative particles orbiting the central nucleus
Rutherford's model shows the positive charge of an atom as a very small area
Thomson's model shows at sea of negative charged particles surrounding a small, positive area

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 12:00, ameliaxbowen7
Suppose a comet has an orbital period of 309.1 years around the sun. what is it’s average distance from the sun?
Answers: 1


Physics, 23.06.2019 04:00, abelinoperez652
Acaregiver's love, affection, and acceptance are considered conditional if they're given only when the child is well-behaved or meets set expectations. t or f
Answers: 3
You know the right answer?
Atomic models have changed over the decades. Two early atomic models can be seen here. There is a dr...
Questions in other subjects:



English, 08.10.2020 01:01

English, 08.10.2020 01:01






Mathematics, 08.10.2020 01:01



