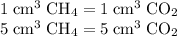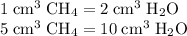Physics, 16.01.2021 18:30 schapethan
A mixture of 5 cm3 of CH4 and 100 cm3 of air is exploded. Assume air is 80% N2 by volume and 20% O2 by volume. The resulting mixture is cooled. All volumes are measured at room temperature and pressure.
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
What is the composition of the resulting gas?

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 05:30, alexportillo859
Blank liquids and gases exert a buoyant force on objects placed in them
Answers: 3


Physics, 22.06.2019 14:10, kortetsosie8813
Match these items. 1. coulombs __force 2. ohms __emf 3. centimeters __resistance 4. newtons __charge 5. volts __length
Answers: 1

Physics, 22.06.2019 18:00, jeff2852
Astone is thrown with a speed v0 and returns to earth, as the drawing shows. ignore friction and air resistance, and consider the initial and final locations of the stone. which one of the following correctly describes the change δpe in the gravitational potential energy and the change δke in the kinetic energy of the stone as it moves from its initial to its final location? options a.δpe = 0 j and δke = 0 j b.δpe is positive and δke is negative c.δpe = 0 j and δke is positive d.δpe is negative and δke is positive e.δpe = 0 j and δke is negative
Answers: 3
You know the right answer?
A mixture of 5 cm3 of CH4 and 100 cm3 of air is exploded. Assume air is 80% N2 by volume and 20% O2...
Questions in other subjects:


History, 13.02.2020 02:00






English, 13.02.2020 02:00


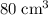 Nitrogen,
Nitrogen, 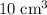 oxygen,
oxygen, 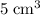 carbon dioxide, and
carbon dioxide, and 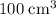 . Thus, the volume of oxygen and nitrogen in the air has been:
. Thus, the volume of oxygen and nitrogen in the air has been: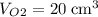
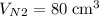
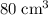 .
.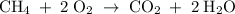
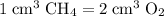
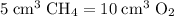
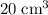 , the remaining oxygen after the reaction has been
, the remaining oxygen after the reaction has been