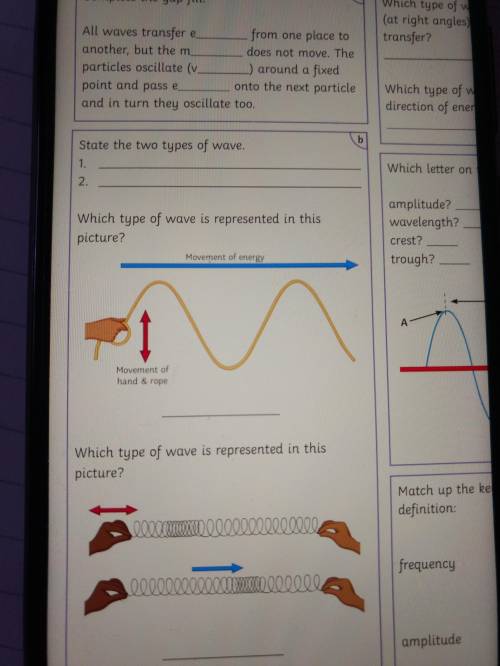
Which type of wave is represented in this picture?
...

Physics, 14.01.2021 14:00 karinahelpz1008
Which type of wave is represented in this picture?

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 06:20, walmartislife
Part 1: a magnetic levitation or maglev train rides rails without touching them. explain how this works using your data. include the appropriate magnet drawing in your answer. part 2: two objects are near a bar magnet. one is about 1 cm away, while the other is 6 cm away. compare and contrast the magnetic force that affects each object. use your data to answer the question
Answers: 1

Physics, 22.06.2019 10:30, Kathryn014
Light from a sodium lamp passes through a diffraction grating that has 1000 slits per millimeter. the interference pattern is viewed on a screen 1.000 m behind the grating. the first (m = 1) two bright yellow fringes that are visible are 0.7288 m and 0.7300 m from the central maximum. what are the wavelengths of these two fringes?
Answers: 2


Physics, 22.06.2019 14:30, 21schraderlily
Will mark as brainliest how does a catapult increase the trajectory of an object? ps. answer as if u were a 5th grader
Answers: 1
You know the right answer?
Questions in other subjects:



History, 23.04.2020 01:41

Physics, 23.04.2020 01:41


Mathematics, 23.04.2020 01:41

Health, 23.04.2020 01:41





