
Physics, 12.01.2021 08:40 mmvill0809
A 300.0 g sample of water at 80.0°C is mixed with 300.0 g of water at
10.0°C. Assuming no heat loss to the surroundings, what is the final
temperature of the mixture? The specific heat capacity of liquid water is
4180 J/kg.°C *
Will give brainliest and lot of stars please help

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 22:30, tladitidimatso1783
A2 kg ball travellng to the right with a speed of 4 m/s collidees with a 5 kg ball traveling to the left with a speed of 3 m/s. take right to be the positive direction. what is the total momentum of the two balls before they collide? what is the total momentum of the two balls after they collide?
Answers: 1


Physics, 22.06.2019 13:30, sukiyoshi10
Global warming will produce rising sea levels partly due to melting ice caps but also due to the expansion of water as average ocean temperatures rise. to get some idea of the size of this effect, calculate the change in length of a column of water 1.00 km high for a temperature increase of 1.00ºc. note that this calculation is only approximate because ocean warming is not uniform with depth. (answer in ×10^{-3} −3 m)
Answers: 1

Physics, 22.06.2019 19:30, dragador7601
Which of the following compounds would be primarily ionic? methane, ch4, ammonia nh3, calcium chloride, cacl2, or carbon dioxide, co2
Answers: 1
You know the right answer?
A 300.0 g sample of water at 80.0°C is mixed with 300.0 g of water at
10.0°C. Assuming no heat loss...
Questions in other subjects:






Computers and Technology, 29.08.2020 01:01




Mathematics, 29.08.2020 01:01

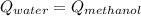
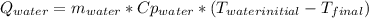
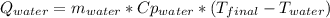
![0.3*4180*(80-T_{final})=0.3*4180*(T_{final}-10)\\100320-1254*T_{final}=1254*T_{final}-12540\\112860=2508*T_{final}\\T_{final}=45[C]](/tpl/images/1028/6130/52afa.png)


