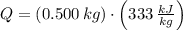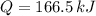Physics, 06.01.2021 14:00 dillon100097
A sample of 0.500 kg of ice is held at a temperature of 0°C. How much energy
must be added to completely turn the ice into liquid water? (The latent heat of
vaporization for water is 2260 kJ/kg; the latent heat of fusion for water is 333
kJ/kg.)
A. 1130 kJ
B. 666 kJ
C. 167 kJ
D. 4520 kJ

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 01:30, chandranewlon
Ablock of mass 1.5 kg slides down an inclined plane that has an angle of 15. if the inclined plane has no friction and the block starts at a height of 3 m, how much kinetic energy does the block have when it reaches the bottom? acceleration due to gravity is g = 9.8 m/s2. a. 6.8 j b. 50.9 j c. 0 j d. 44.1 j
Answers: 1


Physics, 22.06.2019 11:30, tommyaberman
You've already seen the value of 9.8 in this lesson. what's this value called? what quantity does it represent?
Answers: 2
You know the right answer?
A sample of 0.500 kg of ice is held at a temperature of 0°C. How much energy
must be added to compl...
Questions in other subjects:

Arts, 12.12.2020 17:00





English, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

Spanish, 12.12.2020 17:00

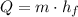 (1)
(1)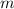 - Mass of ice, measured in kilograms.
- Mass of ice, measured in kilograms. 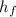 - Latent heat of fusion, measured in kilojoules per kilogram.
- Latent heat of fusion, measured in kilojoules per kilogram. 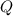 - Latent heat, measured in kilojoules.
- Latent heat, measured in kilojoules.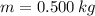 and
and 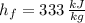 , the latent heat of ice is:
, the latent heat of ice is: