
Physics, 03.12.2020 17:00 juanitarodriguez
An air bubble released by a deep-water diver, 115 m below the surface of a lake, has a volume of 1.60 cm3. The surface of the lake is at sea level, and the density of the lake water can be approximated as that of pure water. As the bubble rises to the surface, the temperature of the water and the number of air molecules in the bubble can each be approximated as constant. Find the volume (in cm3) of the bubble just before it pops at the surface of the lake. ___ cm3.

Answers: 1


Other questions on the subject: Physics



Physics, 22.06.2019 17:20, lilzaya510
Properties seen when one one substance changes to another are known as properties
Answers: 1

Physics, 22.06.2019 17:50, arunamvr
Which of the following best describes internal energy? a. the difference between the kinetic and potential energies of the particles in a system b. the sum of the kinetic and potential energies of the particles in a system c. the sum of the kinetic and thermal energies of the particles in a system d. the difference between the kinetic and thermal energies of the particles in a system
Answers: 2
You know the right answer?
An air bubble released by a deep-water diver, 115 m below the surface of a lake, has a volume of 1.6...
Questions in other subjects:

Biology, 24.08.2019 16:10

Mathematics, 24.08.2019 16:10

Mathematics, 24.08.2019 16:10


English, 24.08.2019 16:10

English, 24.08.2019 16:10



History, 24.08.2019 16:10


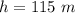
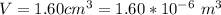


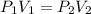
 is the pressure of the bubble at the depth where it is released which i mathematically represented as
is the pressure of the bubble at the depth where it is released which i mathematically represented as 
 is the atmospheric pressure with value
is the atmospheric pressure with value 
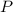 is the pressure due to the depth which is mathematically represented as
is the pressure due to the depth which is mathematically represented as 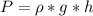
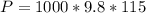

 is the density of pure water with value
is the density of pure water with value 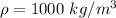

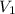 is the volume of the bubble at the depth where it is released
is the volume of the bubble at the depth where it is released is the pressure of the bubble at the surface which is equivalent to the atmospheric temperature
is the pressure of the bubble at the surface which is equivalent to the atmospheric temperature  is the volume of the bubble at the surface
is the volume of the bubble at the surface 




