
Physics, 02.12.2020 20:00 SoWhat2237
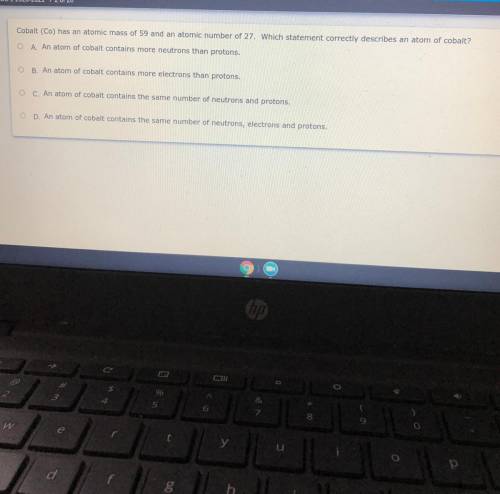
Cobalt (Co) has an atomic mass of 59 and an atomic number of 27. Which statement correctly describes an atom of cobalt?
O A. An atom of cobalt contains more neutrons than protons.
B. An atom of cobalt contains more electrons than protons.
O C. An atom of cobalt contains the same number of neutrons and protons.
O D. An atom of cobalt contains the same number of neutrons, electrons and protons.

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 18:30, aaliyahmaile13
What is the conversion factor between kg/m^3 and g/m^3. express your experimental values for the densities in kg/m^3.
Answers: 1

Physics, 21.06.2019 22:30, leannamat2106
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Physics, 22.06.2019 01:00, hoshoronline8454
When you turn on a battery-operated device, an electrical circuit is
Answers: 1

Physics, 22.06.2019 04:20, pippalotta
Astone is thrown into a pond. what happens to the amplitude of the resulting waves as they get farther from the point where the stone hit the water? explain.
Answers: 3
You know the right answer?
Cobalt (Co) has an atomic mass of 59 and an atomic number of 27. Which statement correctly describes...
Questions in other subjects:












