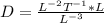When we do dimensional analysis, we do something analogous to stoichiometry, but with multiplying instead of adding. Consider the diffusion constant that appears in Fick's first law:
J= -Ddn/dx
In this expression, J represents a flow of particles: number of particles per unit area per second, n represents a concentration of particles: number of particles per unit volume; and x represents a distance. We can assume that they have the following dimensionalities:
[J] = 1/L2T
[n] = 1/L3
[x] = L
Required:
From this, determine the dimensionality of D.

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 23:30, xxxamslashxxx9
If 21.2 kcals of energy is released by this reaction, how many kj does the reaction release? (1 cal 4.184 j)
Answers: 1

Physics, 22.06.2019 03:00, natasniebow
Which of the following harmful chemicals are found in tobacco smoke? a. carbon monoxide b. carbon dioxide c. nicotine b. carbon dioxide d. both a and c
Answers: 2

Physics, 22.06.2019 06:40, alicemareus
Determine the change in width a, height b, thickness t when a plate is subjected to the uniform distributed load and is made of material having modulus of elasticity e=230 gpa and poisson's ratio ν=1/3. given : a=400 mm and b= 300 mm also the uniformly distributed load in downward y direction of plate is 2 mn/m and in the positive x direction is 3 mn/m and t=20 mm
Answers: 1
You know the right answer?
When we do dimensional analysis, we do something analogous to stoichiometry, but with multiplying in...
Questions in other subjects:





Mathematics, 08.11.2019 05:31


Mathematics, 08.11.2019 05:31

Biology, 08.11.2019 05:31

Chemistry, 08.11.2019 05:31

Mathematics, 08.11.2019 05:31

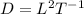
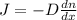
![[J] = \frac{1}{L^2 T}](/tpl/images/0806/8188/0107d.png)
![[n] =\frac{1}{L^3}](/tpl/images/0806/8188/7ef2c.png)
![[x] = L](/tpl/images/0806/8188/a9174.png)
![\frac{1}{L^2 T} = -D \frac{d(\frac{1}{L^3})}{d[L]}](/tpl/images/0806/8188/f5d32.png)
![\frac{1}{L^2 T} = -D \frac{(\frac{1}{L^3})}{[L]}](/tpl/images/0806/8188/42ff8.png)