
Physics, 08.10.2020 01:01 ashtyndowdle
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited states to energy level 2 (n1). Use the Rydberg equation and your measured wavelengths to determine the energy transitions associated with each of your observed wavelengths for hydrogen. In other words, calculate the excited state energy level (n2) for each of your observed wavelengths for hydrogen. n has integer values; so, calculate it first with appropriate significant digits, then round it to an integer. Use the key to show your work for at least one calculation. Must show energy levels for each hydrogen wavelength.

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 02:00, evarocks352
How does the amount of energy required to hold each proton and neutron in the nucleus compare to the energy released when they are removed?
Answers: 3


Physics, 22.06.2019 23:40, ttwright24
Which type of energy is transferred by convection currents?
Answers: 2

Physics, 23.06.2019 09:20, hannahkharel2
How does an increase in temperature generally affect the rate of a reaction?
Answers: 3
You know the right answer?
2. The visible region of the hydrogen spectrum results from relaxation of electrons from excited sta...
Questions in other subjects:

Biology, 31.01.2020 05:54

Biology, 31.01.2020 05:54

Chemistry, 31.01.2020 05:54


English, 31.01.2020 05:54

Biology, 31.01.2020 05:54

Physics, 31.01.2020 05:54


Biology, 31.01.2020 05:54

Mathematics, 31.01.2020 05:54

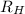 (1/2² - 1 / n²)
(1/2² - 1 / n²)


