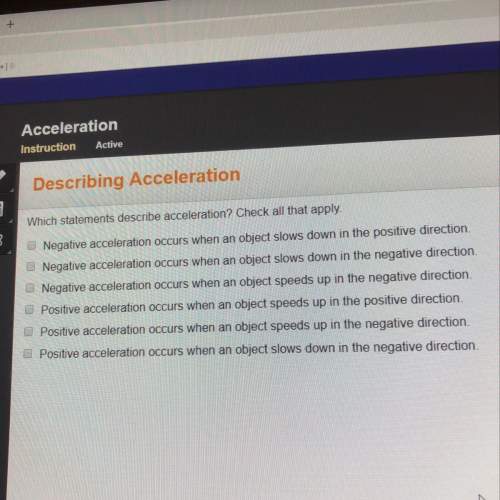
The modern model of the atom describes electrons in a little less specific detail than earlier models did. Why is it that being less sure about the placement of electrons in an atom is actually an improvement over earlier models?
Electrons do not follow specific paths, so describing the area where an electron is likely to be is more scientifically accurate.
Electrons were thought to be negatively charged, but now scientists know that their charge depends on their energy level.
The modern model is based on mathematical equations, so the results become less clear when the decimals are rounded.
The position of electrons in atomic models has changed so much that it is better to have a less specific model than to be wrong again.

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 21:30, genyjoannerubiera
Ahydroelectric plant takes energy from water and turns it into electrical energy. what are the transformations of energy in the water molecules that are used in the process of generating electricity this way?
Answers: 3

Physics, 21.06.2019 23:00, laurachealsy923
How many dots must be added to the symbol to properly represent a standard nitrogen ion? a) 1 b) 3 c) 5 d) 8
Answers: 1


Physics, 22.06.2019 11:30, smilequi9653
Considering only the earth's rotation, determine how much later the asteroid would have had to arrive to put the explosion above helsinki at longitude 25˚ e? this would have obliterated the city.
Answers: 1
You know the right answer?
The modern model of the atom describes electrons in a little less specific detail than earlier model...
Questions in other subjects:

Chemistry, 30.03.2020 20:21






Mathematics, 30.03.2020 20:21



Physics, 30.03.2020 20:22