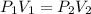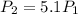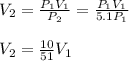Physics, 12.08.2020 04:01 Mtovar7713
an ideal gas is confined to a container with adjustable volume. the number of moles, n, and temperature, t, are constant. by what factor will the volume change if pressure increase by a factor of 5.1

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 19:30, jonquil201
The us government wants to allocate billions of dollars in the next 10 years to assure our future energy security. the funds will be spread among a variety of possible energy resources. where do you think the government should put the greatest support: solar energy, wind energy, clean coal, oil exploration, gas exploration, or a combination of sources? are there other efforts that should be explored? support your position with cited information for both questions.
Answers: 2

Physics, 22.06.2019 04:30, jakiyahporter0817
Asystem containing an ideal gas at a constant pressure of 1.22×10^5 pa gains 2140 j of heat. during the process, the internal energy of the system increases by 2320 j. what is the change in volume of the gas?
Answers: 3

Physics, 22.06.2019 17:30, giraffegurl
Distinguish between linear momentum and angular momentum
Answers: 2

Physics, 22.06.2019 21:30, mxrvin4977
Ineed this now one scientist feels that the changes in global climatic patterns may be good for the health of the biosphere. another scientist feels that sharp changes in climatic patterns could result in the extinction of the biosphere. which of these is the most likely reason the two scientists gave conflicting opinions on the impact of changes in the global climatic pattern? they do not have enough knowledge on the topic. they did not discuss the issue among themselves. they believe conflicting opinion strengthens science. they study different areas of science and have different scientific focuses.
Answers: 1
You know the right answer?
an ideal gas is confined to a container with adjustable volume. the number of moles, n, and temperat...
Questions in other subjects:

Arts, 12.07.2019 12:00

English, 12.07.2019 12:00

History, 12.07.2019 12:00

Spanish, 12.07.2019 12:00

Social Studies, 12.07.2019 12:00

Chemistry, 12.07.2019 12:00



Mathematics, 12.07.2019 12:00