
Physics, 30.07.2020 01:01 santosbeti90
Salt compounds are commonly used to melt ice that forms on sidewalks in the winter. A common chemical that is used to melt sidewalk ice is calcium chloride, CaCl2(s). When calcium chloride dissolves into solution is releases thermal energy which aides in melting
the ice.
The molar mass of water is 18.02 g/mol
The thermal energy, in kilojoules (kJ) that must be released from the calcium chloride,
CaCl2(s), to melt 10.0 kg of ice, expressed in scientific notation is a. bc x 104 k).
46
55
The values of a, b, c, and d.


Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 07:00, hdjsjfjruejchhehd
If a tank filled with water contains a block and the height of the water above point a within the block is 0.8 meter, what is the pressure at point a?
Answers: 2


You know the right answer?
Salt compounds are commonly used to melt ice that forms on sidewalks in the winter. A common chemica...
Questions in other subjects:

Mathematics, 27.03.2021 23:40

Arts, 27.03.2021 23:40

Chemistry, 27.03.2021 23:40




Business, 27.03.2021 23:40


Mathematics, 27.03.2021 23:50

Mathematics, 27.03.2021 23:50

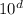 kJ
kJ

