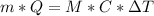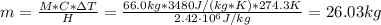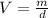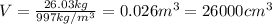The evaporation of sweat is an important mechanism for temperature control in some warm-blooded animals.
a. What mass of water must evaporate from the skin of a 66.0 kg man to cool his body 1.30 °C? The heat of vaporization of water at body temperature (37.0 ∘C) is 2.42×10^6J/kg. The specific heat capacity of a typical human body is 3480 J/(kg⋅K).
b. What volume of water must the man drink to replenish the evaporated water? Compare this result with the volume of a soft-drink can, which is 355 cm^3

Answers: 2


Other questions on the subject: Physics


Physics, 22.06.2019 03:50, cyberdac01
Which statement correctly describes a step-up transformer? a. it increases the amount of electric energy available. b. it increases the voltage of an electric current. c. it changes direct current into alternating current. d. it has more loops of wire in its primary coil.
Answers: 1

Physics, 22.06.2019 12:40, live4dramaoy0yf9
Find the equation for the plane through upper p 0 left parenthesis negative 4 comma negative 8 comma negative 5 right parenthesis perpendicular to the following line. xequalsnegative 4 minus t, yequalsnegative 8 plus 2 t, zequals3 t, minusinfinityless thantless thaninfinity
Answers: 2
You know the right answer?
The evaporation of sweat is an important mechanism for temperature control in some warm-blooded anim...
Questions in other subjects:


Mathematics, 01.09.2021 05:10

Business, 01.09.2021 05:10


Mathematics, 01.09.2021 05:10

Mathematics, 01.09.2021 05:10



English, 01.09.2021 05:10