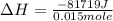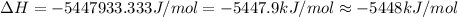Physics, 04.07.2020 14:01 emily200705
The energy from 0.015 moles of octane was used to heat 250 grams of water. The temperature of the water rose from 293.0 K to 371.2 K. What is the enthalpy of combustion of octane? The specific heat capacity of water is 4.18 J/K g.
A. -1226 kJ/mol
B. -5448 kJ/mol
C. 293.25 kJ/mol
D. 1226 kJ/mol

Answers: 3


Other questions on the subject: Physics



You know the right answer?
The energy from 0.015 moles of octane was used to heat 250 grams of water. The temperature of the wa...
Questions in other subjects:

English, 27.10.2020 05:30

Mathematics, 27.10.2020 05:30

Health, 27.10.2020 05:30

English, 27.10.2020 05:30

Mathematics, 27.10.2020 05:30



Computers and Technology, 27.10.2020 05:30

Mathematics, 27.10.2020 05:30

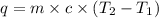
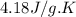
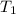 = initial temperature of water = 293.0 K
= initial temperature of water = 293.0 K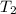 = final temperature of water = 371.2 K
= final temperature of water = 371.2 K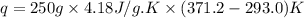
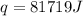
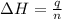
 = enthalpy of combustion of octane = ?
= enthalpy of combustion of octane = ?