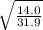Physics, 18.06.2020 18:57 SupremeNerdx
Fill in the blanks for the following: Arigid container of volume 100.0 Liters contains Oxygen gas. It is at room temperature (293 Kelvin), and is at atmospheric pressure (absolute pressure, meaning the gauge pressure is zero). Therefore, the number of moles of Oxygen molecules . Also, the rms-velocity inside is of these Oxygen molecules is most nearly , which is the rms-speed of the Nitrogen molecules just outside the container (the rigid container and its surroundings are in thermal equilibrium).

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 07:30, babygreg2001p97abr
Boxing gloves are padded to lessen the force of a blow. (a) calculate the force exerted by a boxing glove on an opponent’s face, if the glove and face compress 7.50 cm during a blow in which the 7.00-kg arm and glove are brought to rest from an initial speed of 10.0 m/s. (b) calculate the force exerted by an identical blow in the gory old days when no gloves were used and the knuckles and face would compress only 2.00 cm. (c) discuss the magnitude of the force with glove on. does it seem high enough to cause damage even though it is lower than the force with no glove?
Answers: 1

Physics, 22.06.2019 18:10, victoriakraus6599
A200-n force is applied to the foot-operated air pump. the return spring s exerts a 2.6-n·m moment on member oba for this position. determine the corresponding compression force c in the cylinder bd. if the diameter of the piston in the cylinder is 40 mm, estimate the air pressure generated for these conditions. state any assumptions. enter a positive number for the compression force c.
Answers: 2

Physics, 22.06.2019 22:20, destiniout04231
A10-kg piece of aluminum sits at the bottom of a lake, right next to a 10-kg piece of lead, which is much denser than aluminum. which one has the greater buoyant force on it? explain. b - the aluminum a) both have the same buoyant force. b) the aluminum c) the lead d) it cannot be determined without knowing their volumes.
Answers: 1

Physics, 23.06.2019 00:20, dragongacha777
Ahypothetical metal has an orthorhombic unit cell for which the a, b, and c lattice parameters are 0.413 nm, 0.665 nm, and 0.876 nm, respectively. (a) if there are 8 atoms per unit cell and the atomic packing factor is 0.536, determine the atomic radius (in nm). (b) if the density is 3.99 g/cm3, calculate the metal's atomic weight (in g/mol).
Answers: 3
You know the right answer?
Fill in the blanks for the following:
Arigid container of volume 100.0 Liters contains Oxygen gas....
Questions in other subjects:

Spanish, 01.12.2020 18:40


Chemistry, 01.12.2020 18:40



History, 01.12.2020 18:40

Mathematics, 01.12.2020 18:40


Mathematics, 01.12.2020 18:40

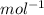

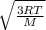
 = 478.6 m/s
= 478.6 m/s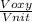 =
= 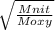
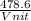 =
=