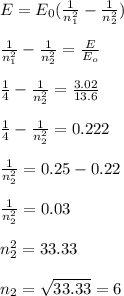Physics, 29.05.2020 00:03 kutemigos9211
MODERN PHYSICS
A photon emitted from an excited hydrogen atom has an energy of 3.02 electron volts. Which electron energy-level transition would produce this photon?
A. n=1 to n=6
B. n=2 to n=6
C. n=6 to n=1
D. n=6 to n=2
I chose B but the correct answer is D can someone tell me why? And what's the difference?

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 23:30, kennydenny4897
Ais not a compound machine. a. bolt b. shovel c. handcart (dolly) d. see-saw (teeter totter)
Answers: 2

Physics, 22.06.2019 15:30, jjjjjj4999
Match each scenario to the form of energy it represents
Answers: 2

Physics, 22.06.2019 17:30, moneyyfletcher
Atruck driver is attempting to deliver some furniture. first, he travels 8 km east, and then he turns around and travels 3 km west. finally, he turns again and travels 13 km to his destination. what is the drivers total displacement?
Answers: 1

Physics, 22.06.2019 21:20, Animallover100
An electron is ejected into a horizontal uniform e⃗ field at a parallel horizontal velocity of v0. assume the electron's initial position x0, initial velocity v0, time t, magnitude of electric field e, electron's mass m, and the magnitude of the electron's charge |e|. ignore the force that earth exerts on the electron. assume the e⃗ field is in the same direction as the initial velocity. part a define the equation for the electron's velocity. express your answer in terms of the variables v0, |e|, t, e, and m.
Answers: 3
You know the right answer?
MODERN PHYSICS
A photon emitted from an excited hydrogen atom has an energy of 3.02 elec...
A photon emitted from an excited hydrogen atom has an energy of 3.02 elec...
Questions in other subjects:

History, 21.12.2019 12:31

Chemistry, 21.12.2019 12:31


Mathematics, 21.12.2019 12:31


Business, 21.12.2019 12:31

History, 21.12.2019 12:31


English, 21.12.2019 12:31

Mathematics, 21.12.2019 12:31