
You put m1 = 1 kg of ice cooled to -20°C into mass m2 = 1 kg of water at 2°C. Both are in a thermally insulated chamber. For water L = 3.33 x 105 J/kg. The specific heat of ice is 2090 J/(kg°C) and of water 4186 J/(kg°C). A. Everything turns to ice at a temperature below 0°C. B. Everything melts and is at a temperature above 0°C. C. There is a mixture of water and ice as the final state. D. The water and ice never reach the same temperature. E. There is not enough information to find the final temperature.

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 15:00, zoriahmendoza
Does this organism still meet the definition of a eukaryote ? why or why not?
Answers: 2

Physics, 22.06.2019 05:00, masie03
Red light strikes a metal surface and electrons are ejected. if violet light is now used with a 10% greater intensity, what will happen to the ejection rate (number of ejected electrons per second) and the maximum energy of the electrons? a) greater ejection rate; same maximum energyb) same ejection rate; greater maximum energyc) greater ejection rate; greater maximum energyd) same ejection rate; same maximum energye) none of the above answers are correct
Answers: 1

Physics, 22.06.2019 05:50, ladyty2109
Astudent wears sunglasses to her read a book more easily which wave interaction occurs as light passes from the air into the plastic lenses? a polarization b. refraction c. diffraction d. interference
Answers: 1

Physics, 22.06.2019 12:30, zayeboyd4971
Uppose we consider the system of the three capacitors as a single "equivalent" capacitor. given the charges of the three individual capacitors calculated in the previous part, find the total charge qtot for this equivalent capacitor. express your answer in terms of v and c.
Answers: 2
You know the right answer?
You put m1 = 1 kg of ice cooled to -20°C into mass m2 = 1 kg of water at 2°C. Both are in a thermall...
Questions in other subjects:



Mathematics, 01.08.2019 12:50

Mathematics, 01.08.2019 12:50

Physics, 01.08.2019 12:50



Physics, 01.08.2019 12:50


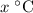 be the final temperature of the mixture. Consider: what are some of the possible values of
be the final temperature of the mixture. Consider: what are some of the possible values of  ? There are three possible outcomes:
? There are three possible outcomes: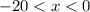 , the final mixture contains only ice.
, the final mixture contains only ice. , the final mixture contains only water.
, the final mixture contains only water. , the final mixture contains both ice and water.Assumption 1
, the final mixture contains both ice and water.Assumption 1 , the energy released when
, the energy released when  of water is cooled from
of water is cooled from 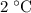 to
to 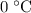 .
. , the energy released when
, the energy released when  , the energy released when
, the energy released when  , where
, where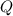 is the energy change due to a change in temperature.
is the energy change due to a change in temperature. is the specific heat capacity.
is the specific heat capacity.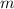 is the mass of the object, and
is the mass of the object, and is the change in temperature. Note that
is the change in temperature. Note that 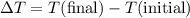 .
. .
. would be negative. Add a minus sign to make sure that the value of
would be negative. Add a minus sign to make sure that the value of 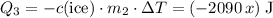 .
.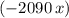 would be positive.
would be positive. the latent heat of fusion of water.
the latent heat of fusion of water. .
. .
. to
to  .
. .
.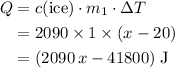 .
. .
.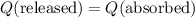 . Therefore:
. Therefore: .
. .
. 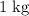 of ice
of ice  .
. .
.
 .
.  , and the mixture will contain both ice and water.
, and the mixture will contain both ice and water.

