
Physics, 07.05.2020 12:02 Lenaaa2019
Once you’ve identified the initial and final conditions, you’re ready to solve for the unknown quantity in your problem. Boyle’s Law expresses the pressure-volume relationship as P1V1=P2V2, so you will need to solve this equation for the unknown quantity and then plug in your known values to calculate the unknown. What pressure would it take to compress 250. L of helium gas initially at 1.00 atm into a 2.00 L tank at constant temperature? Express your answer with the appropriate units.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 00:30, Waakkaa
Glass is transparent to visibile light under normal conditions; however, at extremely high intensities, glass will absorb most of the light incident upon it. this works through a process known as multiphoton absorption. in this process, several photons are absorbed at the same time. if very intense light whose photons carry 2ev of energy is shined onto a material with a band gap of 4ev, that light can be absorbed through two-photon absorption, because two photons have the right amount of energy to bridge the band gap. what is the minimum number of photons of 800-nm light that are needed to equal or exceed the band gap of fused silica glass
Answers: 1

Physics, 22.06.2019 08:30, 2021arabellacorsino
An object weigh 40n in air ,weigh 20n when submerged in water, and 30n when submerged in a liquid of unknown liquid density. what is the density of unknown of liquid?
Answers: 1

Physics, 22.06.2019 09:30, ryanmorse01
The necleus of an atom is made up of what subatomic particles?
Answers: 1

Physics, 22.06.2019 10:50, afonseca73
Asheet of steel 1.5 mm thick has nitrogen atmospheres on both sides at 1200oc and is permitted to achieve a steady-state diffusion condition. the diffusion coefficient for nitrogen in steel at this temperature is 6 x 10- 11 m2 /s, and the diffusion flux is found to be 1.2 x 10-7 kg/m2 -s. also, it is known that the concentration of nitrogen in the steel at the high-pressure surface is 4 kg/m3 . how far into the sheet from this high-pressure side will the concentration be 2.0 kg/m3 ? assume a linear concentration profile.
Answers: 3
You know the right answer?
Once you’ve identified the initial and final conditions, you’re ready to solve for the unknown quant...
Questions in other subjects:

History, 11.12.2019 05:31


Mathematics, 11.12.2019 05:31


Mathematics, 11.12.2019 05:31

Mathematics, 11.12.2019 05:31


Law, 11.12.2019 05:31

Mathematics, 11.12.2019 05:31

Mathematics, 11.12.2019 05:31

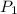 = 1 atm
= 1 atm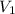 = 250L
= 250L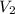 =2L
=2L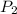 =?
=?

