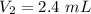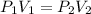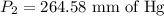A gas is placed in a 5.0-mL syringe. It exerts 127 mm Hg of pressure on the inside walls of the syringe. The syringe's plunger is pressed, reducing the volume of the syringe to 2.4 mL. The cap was not removed from the syringe, so none of the gas escapes. Assuming the temperature of the gas does not change, use Boyle's law to determine the pressure of the compressed gas.

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 23:30, schapethan
In order for the bug to fly through the air a force has to push the bug forward. identify this force. how does the bug produce
Answers: 1

Physics, 22.06.2019 01:30, kayleetruman
The passing of heat through a material while the material itself stays in place. a. radiation b. conduction c. convection
Answers: 2

Physics, 22.06.2019 07:00, divadebbgirl1
Examine the equation. 23490th→23088ra+42he what kind of barrier would you need to block the radioactive particles from this reaction? a. a piece of paper b. a sheet of aluminum foil c. a two-inch block of lead d. a solid concrete block
Answers: 1

Physics, 22.06.2019 11:10, livichavezp2kcd3
Assume this car is driven off a cliff . how many arrows of force need to be drawn in the free body diagram? assume no air resistance -five -one -three -four
Answers: 1
You know the right answer?
A gas is placed in a 5.0-mL syringe. It exerts 127 mm Hg of pressure on the inside walls of the syri...
Questions in other subjects:




Social Studies, 27.06.2019 04:30



English, 27.06.2019 04:30



Mathematics, 27.06.2019 04:30