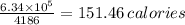Physics, 22.04.2020 04:45 ashleyivers3
In exercising, a weight lifter loses 0.200 kg of water through evaporation, the heat required to evaporate the water coming from the weight lifter's body. The work done in lifting weights is 1.50 105 J. (a) Assuming that the latent heat of vaporization of perspiration is 2.42 106 J/kg, find the change in the internal energy of the weight lifter. J (b) Determine the minimum number of nutritional calories of food (1 nutritional calorie

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 05:30, alexportillo859
Blank liquids and gases exert a buoyant force on objects placed in them
Answers: 3

Physics, 22.06.2019 10:00, myanniespencer39
Which accurately compares concave and convex lenses?
Answers: 2

Physics, 22.06.2019 11:20, puppylove899
Wave functions describe orbitals in a hydrogen atom. each function is characterized by 3 quantum numbers: n, l, and ml. if the value of n = 2: the quantum number l can have values from to . the total number of orbitals possible at the n = 2 energy level is .
Answers: 3

Physics, 22.06.2019 18:00, ggdvj9gggsc
Atank is filled with an ideal gas at 400 k and pressure of 1.00 atm . part a the tank is heated until the pressure of the gas in the tank doubles. what is the temperature of the gas?
Answers: 3
You know the right answer?
In exercising, a weight lifter loses 0.200 kg of water through evaporation, the heat required to eva...
Questions in other subjects:



Mathematics, 04.06.2020 13:10

English, 04.06.2020 13:10



History, 04.06.2020 13:10


Mathematics, 04.06.2020 13:10