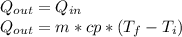reaches an equilibrium temperature of 31.1°C.

Physics, 17.04.2020 06:47 johnisawesome999
A jar of tea is placed in sunlight until it
reaches an equilibrium temperature of 31.1°C.
In an attempt to cool the liquid, which has a
mass of 177 g. 110 g of ice at 0.0°C is added.
At the time at which the temperature of the
tea is 29.1°C, find the mass of the remaining ice in the jar. The specific heat of water is 4186 J/kg.°C. Assume the specific heat capacity of the tea to be that of pure liquid water. Answer in units of g.

Answers: 2


Other questions on the subject: Physics


Physics, 22.06.2019 05:30, kolibeilfuss
What is the most useful equation for electric power?
Answers: 3

Physics, 22.06.2019 21:20, CM0
Abeverage can is made of 3004-h19 aluminum alloy (elastic modulus 69 gpa, tensile yield strength 285 mpa, density 2.72 g/cm^3). the dimensions on the can are approximated as a thin-walled cylinder with a height of 4.83 inches, diameter of 2.60 inches. empty the can has a mass of 14.2 g. determine: a. the wall thickness of the cylinder b. assuming a pinned-pinned condition what is the critical load? c. assuming a fixed-fixed condition what is the critical load?
Answers: 1
You know the right answer?
A jar of tea is placed in sunlight until it
reaches an equilibrium temperature of 31.1°C.
reaches an equilibrium temperature of 31.1°C.
Questions in other subjects:


Mathematics, 12.12.2019 00:31


Mathematics, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31

History, 12.12.2019 00:31