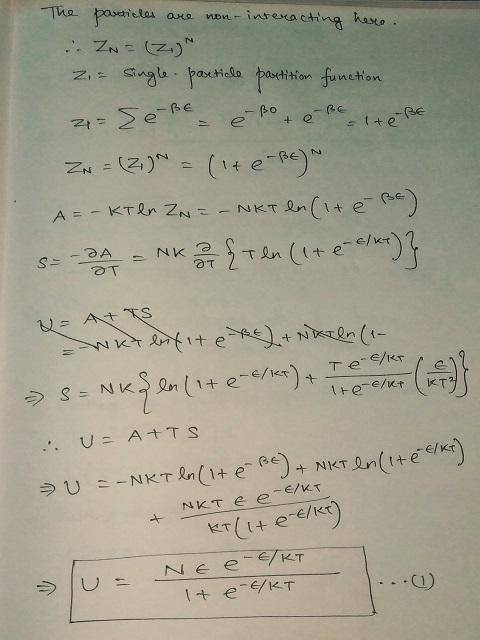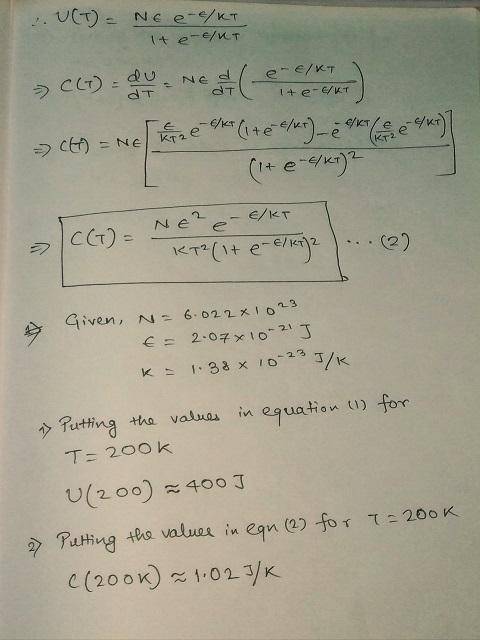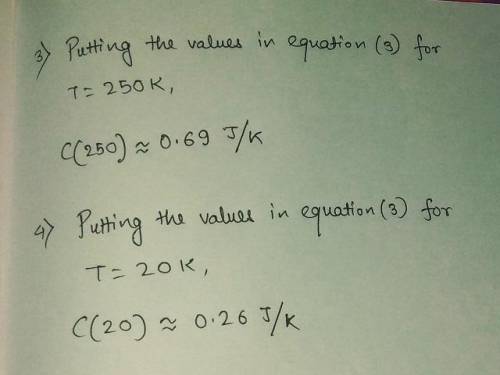Consider N two-state systems at temperature T. All systems are identical, with one state at energy 0 and the other at energy ϵ.
Using the Boltzmann factor for the two states, compute a formula for the energy U(T) as a function of N,ϵ, and T.
Using C(T)=dU/dT, derive a formula for the heat capacity in terms of the same variables.
Use the formulas for U(T) and C(T) to answer the questions below, assuming that N=6.022 × 1023 and ϵ= 2.07 × 10-21 J for all questions:
1) Compute the internal energy U when T-200 K.
2) Compute the heat capacity when T-200 K.
3) Compute the heat capacity when T-250 K.
4) Compute the heat capacity when T-20 K.

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 18:50, gutierrezaandrea56
Study the following reaction carefully. what classification should this reaction have? 4al + 3o2 2al2o3synthesisdecompositionsingle replacementdouble displacement
Answers: 1

Physics, 22.06.2019 02:30, mhurtado143
Agas contained within a piston-cylinder assembly undergoes three processes in series: process 12: compression with pv= constant from 1 bar and 1 liter to 4 bar. process 23: constant pressure expansion to 1 liter. process 31: constant volume calculate the pressure and volume at each state, and sketch the processes on a p-vdiagram labeled with pressure and volume values at each numbered stat
Answers: 2

Physics, 22.06.2019 04:20, livigrace9004
Calculate the capacitance of a system that stores 2.0 x 10^-10c of charge at 100.0 v. use c=q/v. a. 2.0 x 10^-12 f b. 2.0 x 10^-8 f c. 5.0 x 10^11 f d. 5.0 x 10^7 f
Answers: 1
You know the right answer?
Consider N two-state systems at temperature T. All systems are identical, with one state at energy 0...
Questions in other subjects:



Chemistry, 15.01.2021 22:20



Mathematics, 15.01.2021 22:20

Mathematics, 15.01.2021 22:20



Biology, 15.01.2021 22:20