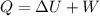Which of the following statements is supported by the first law of thermodynamics?
A The ener...

Which of the following statements is supported by the first law of thermodynamics?
A The energy inside any system is equal to the energy outside.
B The total amount of energy in the universe is steadily decreasing.
C Energy can never enter a closed system, nor can it leave a closed system
D The total energy in a closed system is a conserved quantity.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 03:30, itsRyanPlayzMC9660
Scout feels ill one day before school. her mother puts a thermometer in her mouth and the temperature begins to climb to 100°f. what is happening between the cooler thermometer and scout's body? a) scout's body is applying a force to the particles in the thermometer. b) kinetic energy is being transferred from her mouth to the thermometer. c) potential energy is being lost by the thermometer and gained by scout's body. d) the potential energy stored in foods is converted to mechanical energy to raise the mercury in the thermometer.
Answers: 1

Physics, 22.06.2019 07:30, michaireid04
Identify the theory that can be used to explain each phenomenon. answers diffraction: wave theory interference: wave theory reflection: both particle and wave theories refraction: both particle and wave theories
Answers: 3


Physics, 22.06.2019 22:20, bryanmcmillianjr
Which symbol in the first law of thermodynamics represents the sum of the chemical and thermal energy stored in atoms and molecules? a. q b. w c. v d. u
Answers: 3
You know the right answer?
Questions in other subjects:



Social Studies, 26.08.2019 13:20

History, 26.08.2019 13:20


Mathematics, 26.08.2019 13:20


Mathematics, 26.08.2019 13:20

Business, 26.08.2019 13:20