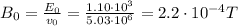Physics, 24.03.2020 21:23 genyjoannerubiera
In the late 19th century, great interest was directed toward the study of electrical discharges in gases and the nature of so-called cathode rays. One remarkable series of experiments with cathode rays, conducted by J. J. Thomson around 1897, led to the discovery of the electron. With the idea that cathode rays were charged particles, Thomson used a cathode-ray tube to measure the ratio of charge to mass, q/m, of these particles, repeating the measurements with different cathode materials and different residual gases in the tube.
Part A
What is the most significant conclusion that Thomson was able to draw from his measurements?
a. He found a different value of q/m for different cathode materials.
b. He found the same value of q/m for different cathode materials.
c. From measurements of q/m he was able to calculate the charge of an electron.
d. From measurements of q/m he was able to calculate the mass of an electron.
Part B
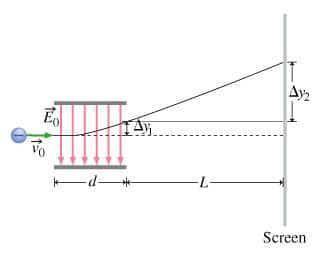
What is the distance Δy between the two points that you observe? Assume that the plates have length d, and use e and m for the charge and the mass of the electrons, respectively.
Express your answer in terms of e, m, d, v0, L, and E0.
Part C
In your experiment, you measure a total deflection of 4.12 cm when an electric field of 1.10×103V/m is established between the plates (with no magnetic field present). When you add the magnetic field, to what value do you have to adjust its magnitude B0 to observe no deflection? Assume that the plates are 6.00 cm long and that the distance between them and the screen is 12.0 cm. Express your answer numerically in tesla.

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 23:10, itzlianne
A248-g piece of copper is dropped into 390 ml of water at 22.6 °c. the final temperature of the water was measured as 39.9 °c. calculate the initial temperature of the piece of copper. assume that all heat transfer occurs between the copper and the water. remember, the density of water is 1.0 g/m
Answers: 1

Physics, 22.06.2019 07:30, sansyboy7891
Quantum mechanics applies to subatomic, atomic, nanometer-size, and micrometer-size systems. nanometer, micrometer, and kilometer-size systems. atomic, nanometer-size, and micrometer-size systems. subatomic, atomic, and nanometer-size systems.
Answers: 2

Physics, 22.06.2019 15:20, alissalhenry
Arigid tank is divided into two equal parts by a partition. one part of the tank contains 3 kg of compressed liquid water at 400 kpa and 60°c while the other part is evacuated. the partition is now removed, and the water expands to fill the entire tank. determine the entropy change of water during this process, if the final pressure in the tank is 40 kpa. use steam tables.
Answers: 3

Physics, 22.06.2019 18:00, Zaydblackwood06
The photo shows sugar dissolved into a solution with excess sugar at the bottom of the jar this type of solution is classified as a. unsaturated b. compound c. saturated d. plasma
Answers: 1
You know the right answer?
In the late 19th century, great interest was directed toward the study of electrical discharges in g...
Questions in other subjects:

Mathematics, 14.03.2020 08:36




Mathematics, 14.03.2020 08:38

Mathematics, 14.03.2020 08:38




English, 14.03.2020 08:39

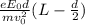
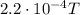

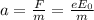
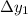 :
: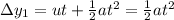
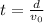
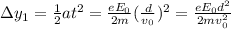
 , while the vertical velocity is
, while the vertical velocity is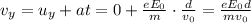
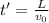
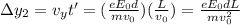
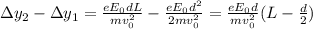 (1)
(1)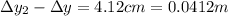
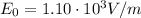 (strength of the electric field)
(strength of the electric field)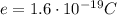 (electron charge)
(electron charge)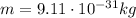 is the mass of the electron
is the mass of the electron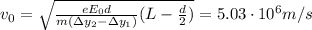
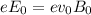
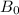 is the magnitude of the magnetic field.
is the magnitude of the magnetic field.