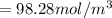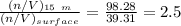Physics, 23.03.2020 16:54 Jamilia561
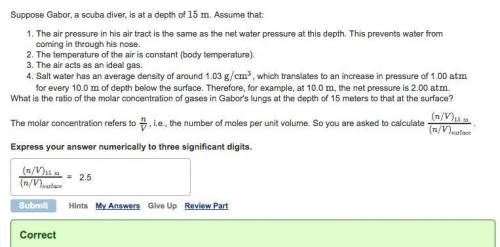
Suppose Gabor, a scuba diver, is at a depth of 15m. Assume that: The air pressure in his air tract is the same as the net water pressure at this depth. This prevents water from coming in through his nose. The temperature of the air is constant (body temperature). The air acts as an ideal gas. Salt water has an average density of around 1.03 g/cm3, which translates to an increase in pressure of 1.00 atm for every 10.0 m of depth below the surface. Therefore, for example, at 10.0 m, the net pressure is 2.00 atm. What is the ratio of the molar concentration of gases in Gabor's lungs at the depth of 15 meters to that at the surface

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 10:10, randlemccray4305
What is the angular momentum of the bar about the axle?
Answers: 3

Physics, 22.06.2019 11:00, Arielledt10
1. jay fills a wagon with sand (about 20 kg) and pulls it with a rope 30 m along the beach. he holds the rope 25° above the horizontal. the rope exerts a 20-n tension force on the wagon. how much work does the rope do on the wagon?
Answers: 1
You know the right answer?
Suppose Gabor, a scuba diver, is at a depth of 15m. Assume that: The air pressure in his air tract i...
Questions in other subjects:

Computers and Technology, 08.07.2019 02:00

Biology, 08.07.2019 02:00


Health, 08.07.2019 02:00

Health, 08.07.2019 02:00

Chemistry, 08.07.2019 02:00


Business, 08.07.2019 02:00

Mathematics, 08.07.2019 02:00

Mathematics, 08.07.2019 02:00

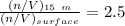
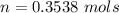
![P_d = [\frac{15m}{10m} ] (1 atm) + 1 atm](/tpl/images/0558/9055/c032e.png)
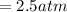
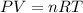


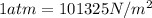 for
for  ,
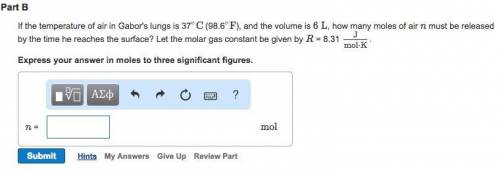
,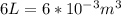 for volume , 8.314 J/mol. K for R , (37° +273) K for T into the equation
for volume , 8.314 J/mol. K for R , (37° +273) K for T into the equation 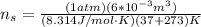
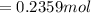

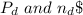 are pressure and no of moles at the depth of the water
are pressure and no of moles at the depth of the water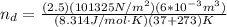
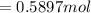
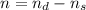

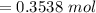
![[\frac{n}{V} ]_{surface} = \frac{0.2359mol}{6*10^-3m^3}](/tpl/images/0558/9055/08954.png)

![[\frac{n}{V} ]_{15m} = \frac{0.5897}{6*10^{-3}}](/tpl/images/0558/9055/d7097.png)