
Physics, 20.03.2020 11:59 elijahbebeastin
The molar heat of fusion of benzene is kJ/mol. Its molar heat of vaporization is kJ/mol. Calculate the heat required to melt g benzene at its normal melting point. Calculate the heat required to vaporize g benzene at its normal boiling point. Why is the heat of vaporization more than three times the heat of fusion?

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 07:30, babygreg2001p97abr
Boxing gloves are padded to lessen the force of a blow. (a) calculate the force exerted by a boxing glove on an opponent’s face, if the glove and face compress 7.50 cm during a blow in which the 7.00-kg arm and glove are brought to rest from an initial speed of 10.0 m/s. (b) calculate the force exerted by an identical blow in the gory old days when no gloves were used and the knuckles and face would compress only 2.00 cm. (c) discuss the magnitude of the force with glove on. does it seem high enough to cause damage even though it is lower than the force with no glove?
Answers: 1

You know the right answer?
The molar heat of fusion of benzene is kJ/mol. Its molar heat of vaporization is kJ/mol. Calculate...
Questions in other subjects:

Mathematics, 26.10.2019 21:43

Advanced Placement (AP), 26.10.2019 21:43

History, 26.10.2019 21:43




Mathematics, 26.10.2019 21:43

Mathematics, 26.10.2019 21:43

Physics, 26.10.2019 21:43

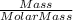 which is
which is 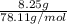 = 0.1056 moles
= 0.1056 moles

