
Physics, 19.03.2020 00:03 jalenshayewilliams
Acetylene gas (C2H2) is used in welding torches. When it reacts with oxygen, it produces carbon dioxide (CO2) and steam (H2O). The reaction can be described by the equation:
2C2H2 + 5O2 ➔ 4CO2 + 2H2O. How much mass of C2H2 is needed to react with 68.1 g of O2 to produce 75.0 g of CO2 and 15.35 g of steam?

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 10:00, jonlandis6
Need people build a dam to create a reservoir that supplies water a nearby city needs. describe two ways this action will likely affect the water cycle in the local environment. (5 points) worth 20 points
Answers: 1


Physics, 22.06.2019 15:10, Pookaapoo8832
When electrons are added to the outermost shell of a carbon atom, it forms--an anion that has a larger anion that has a smaller cation that has a larger cation that has a smaller radius.
Answers: 3
You know the right answer?
Acetylene gas (C2H2) is used in welding torches. When it reacts with oxygen, it produces carbon diox...
Questions in other subjects:

English, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00

Chemistry, 11.12.2020 01:00


Social Studies, 11.12.2020 01:00


Mathematics, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00

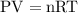 at a constant temperature,
at a constant temperature, 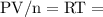 constant. That means,
constant. That means,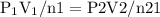
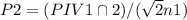
![P 2=(P 1 V 1 \cap 2) /(V 2 n 1)=\left[(155 a t m)^{*}(5.00 L)^{*}(2 m o l e s)\right] /\left[(25.00 L)^{*}(5 \text { moles }]\right]=124 \text { atm }](/tpl/images/0552/9219/932b9.png)


