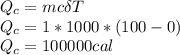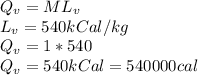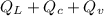The quantity of heat Q that changes the temperature L1Tof a mass mof a substance isgiven by Q = cmt:T, where c is the specific heat capacity of the substance. Forexample, forH20,c=1caljg'C",Andfora change of phase, the quantity of heat Q that changes the phase of a mass m is Q = ml., where L is the heat of fusion or heat of vaporization of the substance. For example, for H20, the heat offusion is 80 cal/g (or 80 kcaljkg) and the heat of vaporization is 540 cal/g (or 540 kcaljkg). Use these relationships to determine the number of calories to change (a) 1 kg ofO°C ice to O°C ice water, (b) 1 kg ofO°C ice water to 1 kg of 100°C boiling water, (c) 1 kg of 100°C boiling water to 1 kg of 100°C steam, and (d) 1 kg ofO°C ice to 1 kg of 100°C steam.

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 08:30, fernandoramirez086
Does anyone know how to solve this problem? i really need . i made an attempt but i just cant get it. a metal rod is 25.000 cm long at 25.0 degrees celsius. when heated to 102.0 degrees celsius, it is 25.054 cm long. what is the coefficient of linear expansion for this metal.
Answers: 3

Physics, 22.06.2019 14:50, lilyrockstarmag
Abar magnet cut in half will form a total ofpoles. a)fourb)eightc)two
Answers: 1

You know the right answer?
The quantity of heat Q that changes the temperature L1Tof a mass mof a substance isgiven by Q = cmt:...
Questions in other subjects:

Mathematics, 05.12.2019 02:31



History, 05.12.2019 02:31


Mathematics, 05.12.2019 02:31

Advanced Placement (AP), 05.12.2019 02:31


Mathematics, 05.12.2019 02:31

Social Studies, 05.12.2019 02:31

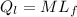 = 1 * 80
= 1 * 80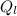 = 80 kCal = 80,000 cal
= 80 kCal = 80,000 cal