Physics, 17.03.2020 03:28 hannahgrac3
A 3.1-mole sample of an ideal gas is gently heated at constant temperature 290 K. The gas expands to 1.5 times its initial volume as its pressure decreases. What is the change in the internal energy of the gas? Let the ideal-gas constant R = 8.314 J/(mol • K).
3000 J
1500 J
0 J
-3000 J

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 21:50, yairreyes01
Adiver in midair has an angular velocity of 6.0 rad/s and a moment of inertia of 1.2 kg·m2. he then pulls is arms and legs into a tuck position and his angular velocity increases to 12 rad/s. the net external torque acting on the diver is zero. what is his moment of inertia in the tuck position?
Answers: 1

Physics, 22.06.2019 09:50, blaqsafire9291
If an athlete runs once around a track, back to the starting line, her average velocity is zero. true or false?
Answers: 3


Physics, 22.06.2019 21:30, Inrimid3619
Aperson touches a large chunk of ice with their hand and remarks, “this is making me cold.” explain what this person is feeling. is the ice transferring “cold” to the person? is there a heat transfer occurring? explain.
Answers: 1
You know the right answer?
A 3.1-mole sample of an ideal gas is gently heated at constant temperature 290 K. The gas expands to...
Questions in other subjects:




Physics, 23.09.2021 15:30


Computers and Technology, 23.09.2021 15:30


Biology, 23.09.2021 15:30

Chemistry, 23.09.2021 15:30

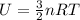
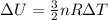
 is the change in temperature of the gas.
is the change in temperature of the gas.