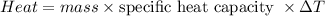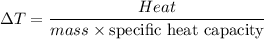Physics, 11.03.2020 02:50 pricilaxo9523
Five-gram samples of copper and aluminum are at room temperature. Both receive equal amounts of energy due to heat flow. The specific heat capacity of copper is 0.09 cal/g°C, and the specific heat capacity of aluminum is 0.22 cal/g°C. Which of the following statements is true?
a. The temperature of each sample increases by the same amount.
b. The aluminum will get hotter than the copper.
c. The copper will get hotter than the aluminum.
d. The temperature of each sample decreases by the same amount

Answers: 2


Other questions on the subject: Physics


Physics, 23.06.2019 00:00, jwmiciche7367
You can melt ice by applying pressure because the solid/liquid line has a slope.
Answers: 3


Physics, 23.06.2019 07:00, school7067
Which best offense of relationship between speed and velocity speed is velocity what displacement velocity and speed with displacement spee is based on a specific direction velocity isis based on displacement
Answers: 1
You know the right answer?
Five-gram samples of copper and aluminum are at room temperature. Both receive equal amounts of ener...
Questions in other subjects:


Biology, 03.08.2020 14:01

Mathematics, 03.08.2020 14:01


Biology, 03.08.2020 14:01