Physics, 10.03.2020 04:37 eymurezgi1
A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake at 15°C. Thermal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Assuming the surroundings to be at 20°C, determine the amount of work that could have been produced if the entire process were executed in a reversible manner.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 12:10, hilljade45
Two forces produce equal torques on a door about the door hinge. the first force is applied at the midpoint of the door; the second force is applied at the doorknob. both forces are applied perpendicular to the door. which force has a greater magnitude?
Answers: 2

Physics, 23.06.2019 00:50, lbelle
Aplayer uses a hockey stick to push a puck at a constant velocity across the ice. the weight of the puck is 170 n. the coefficient of friction is 0.0600. with what force must the player push the puck so that his force just equals the frictional force? 28.3 n 0.999 n 0.102 n 0.0104 n
Answers: 1


Physics, 23.06.2019 02:40, smack2045
An air standard cycle with variable specific heats is executed in a closed system and is composed of the following four processes. 1-2 isentropic compression 100 kpa and 22°c to 600 kpa 2-3 v=constant heat addition to 1500 k 3-4 isentropic expansion to 100 kpa 4-1 p=constant heat rejection to initial state (a) show the cycle on p-v and t-s s (b) calculate the net work output per unit mass (409 kj/kg) (c) determine the thermal efficiency (47.9%)
Answers: 1
You know the right answer?
A 50-kg iron block and a 20-kg copper block, both initially at 80°C, are dropped into a large lake a...
Questions in other subjects:



Mathematics, 19.03.2020 21:50






History, 19.03.2020 21:51

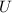 = Δ
= Δ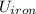 + Δ
+ Δ