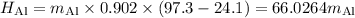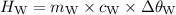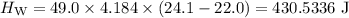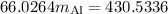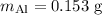A piece of aluminum metal at an initial temperature of 97.3°C was placed in a calorimeter containing 49.0g of water at an initial temperature of 22.0°C. The two were allowed to come to thermal equilibrium and the final temperature was 24.1°C. The specific heats of water and aluminum are 4.184 J/g °C and 0.902 J/g °C, respectively. What was the mass of the Al piece that was added?

Answers: 3


Other questions on the subject: Physics


Physics, 22.06.2019 22:00, marialuizavalen
Which of these is the best way to manage a natural resource
Answers: 3

Physics, 23.06.2019 00:00, alexandria3498
Three resistors are connected in series across a 15-v power supply. if the potential drops across resistors 1 and 2 are 4.1 volts and 3.1 volts, what is the exact potential drop (in volts) across resistor 3?
Answers: 2

Physics, 23.06.2019 00:00, jessicawolfking
What is the difference between mass density and weight density?
Answers: 1
You know the right answer?
A piece of aluminum metal at an initial temperature of 97.3°C was placed in a calorimeter containing...
Questions in other subjects:







History, 03.11.2019 12:31



Mathematics, 03.11.2019 12:31