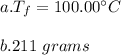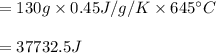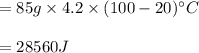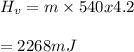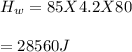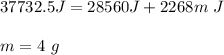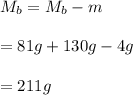You cool a 130.0 g slug of red-hot iron (temperature 745 ∘C) by dropping it into an insulated cup of negligible mass containing 85.0 g of water at 20.0 ∘C. Assume no heat exchange with the surroundings. How do you do this?
Part A What is the final temperature of the water?
Part B What is the final mass of the iron and the remaining water?

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 15:50, Sbudah2937
Electric charge is uniformly distributed inside a nonconducting sphere of radius 0.30 m. the electric field at a point p, which is 0.50 m from the center of the sphere, is 15,000 n/c and is directed radially outward. what is the maximum magnitude of the electric field due to this sphere
Answers: 2

Physics, 22.06.2019 02:30, chycooper101
Aforce of 9.00 newtons acts at an angle of 19.0 to the horizontal. what is its component in the horizontal direction?
Answers: 2


Physics, 22.06.2019 13:00, chie42
Which of the following correctly describes what happens when an atomic bomb explodes? small pieces of fissionable material are joined and form a body with a mass greater than the critical mass, the relative number of neutrons escaping decreases, and a chain reaction and explosion result. large pieces of fissionable matter are brought together quickly and form a body with a mass smaller than the critical mass, the relative number of escaping neutrons increases, and a chain reaction and explosion result.
Answers: 2
You know the right answer?
You cool a 130.0 g slug of red-hot iron (temperature 745 ∘C) by dropping it into an insulated cup of...
Questions in other subjects:



Mathematics, 16.12.2020 19:30


Biology, 16.12.2020 19:30


Social Studies, 16.12.2020 19:30


Chemistry, 16.12.2020 19:30

English, 16.12.2020 19:30