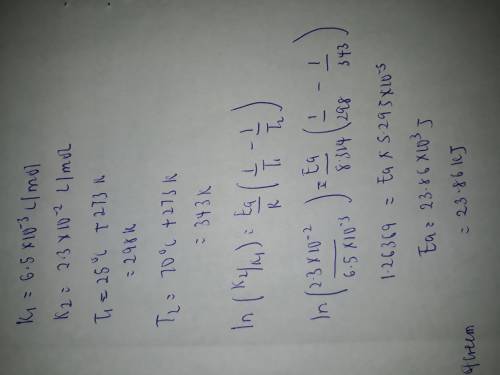Physics, 27.02.2020 03:19 Samonerob2002
A second-order reaction was observed. The reaction rate constant at 25 oC was found to be 6.50 x 10-3L/mol and at 70 oC it was found to be 2.30 x 10-2 L/mol. Calculate the activation energy of this reaction.

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 10:50, madiiiiiii69
If jerome is swinging on a rope and transferring energy from gravitational potential energy to kinetic energy, is being done.
Answers: 3

Physics, 22.06.2019 11:50, kelseybell2707
An electron is traveling with initial kinetic energy k in a uniform electric field. the electron comes to rest momentarily after traveling a distance d. (a) what is the magnitude of the electric field
Answers: 3

Physics, 22.06.2019 14:00, astigall4272
What is the force that opposes motion and works against the downward pull? a) friction b) gravity c) weight d) acceleration
Answers: 1

Physics, 22.06.2019 15:00, tanaemichel
Sodium chloride, nacl, is formed when a sodium atom transfers its electron to a chlorine atom. the difference in charge between the two atoms creates a(n) attraction that bonds them together.
Answers: 1
You know the right answer?
A second-order reaction was observed. The reaction rate constant at 25 oC was found to be 6.50 x 10-...
Questions in other subjects:



Mathematics, 08.04.2021 21:10



History, 08.04.2021 21:10




Computers and Technology, 08.04.2021 21:10