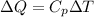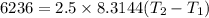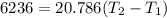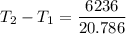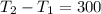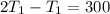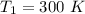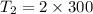Physics, 27.02.2020 02:06 donterriuscollier
2. One mole of a monatomic ideal gas undergoes a reversible expansion at constant pressure, during which the entropy of the gas increases by 14.41 J/K and the gas absorbs 6236 J of thermal energy. Calculate the initial and final temperatures of the gas.

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 05:30, xaviiaquino3378
Choose the most likely outcome of this scenario: jen decided to go bike riding without a helmet. while no one is around during her ride, she is thrown from her bike when her wheel goes into a pothole. she is not injured, but she is terrified to get back on her bike. what happens next? a. her physical health is affected even though she wasn't hurt. b. her mental and emotional health are affected because she is afraid to get back on her bike. c. her social health is affected because she is worried her friends saw the fall. d. her overall health is not affected at all by her fall.
Answers: 1

Physics, 22.06.2019 07:00, Angelanova69134
Photoelectrons with a maximum speed of 6.50 x 107 m/s are ejected from a surface in the presence of light with a frequency of 6.75 x 1014hz. if the mass of an electron is 9.10 x 10-31 kg, calculate in joules the maximum kinetic energy of a single electron. 3.84 x 10-15 j 1.92 x 10-15 j 5.92 x 10-23 j 3.07 x 10-16 j
Answers: 1

Physics, 22.06.2019 16:30, skiddymark3ox93za
Iron is a transition metal with multiple oxidation numbers. (answer the following) a. what is the iron (ii) ion? how does it differ from the iron (iii) ion? b. if iron were to bond with oxygen, predict the formula for each oxidation number of iron. c. how would each formula be named?
Answers: 2
You know the right answer?
2. One mole of a monatomic ideal gas undergoes a reversible expansion at constant pressure, during w...
Questions in other subjects:

Spanish, 10.01.2020 01:31


Computers and Technology, 10.01.2020 01:31

English, 10.01.2020 01:31



English, 10.01.2020 01:31

Mathematics, 10.01.2020 01:31

Biology, 10.01.2020 01:31

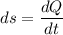
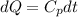
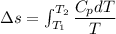
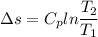
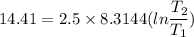
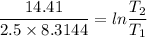
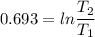
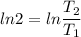
 ...(I)
...(I)