Physics, 26.02.2020 03:54 1341220857
A sample of quartz, which has a specific heat capacity of 0.730 Jg1, is put into a calorimeter (see sketch at right) that contains 300.0 g of water. The quartz sample starts off a insulated 95.8 °C and the temperature of the water starts off at 15.0 °C, when the temperature of the water stops changing it's 17.7 °C. The pressure remains constant at 1 atm.
Calculate the mass of the quartz sample.

Answers: 3


Other questions on the subject: Physics


Physics, 21.06.2019 23:50, george27212
The discovery that the universe appears to be expanding led to a widely accepted theory called a.) the big bang theory b.) the doppler effect c.) hubble’s law d.) solar nebular theory e.) the seyfert theory
Answers: 2

Physics, 22.06.2019 00:10, oktacos
The energy released by a chemical reaction can be measured using a calorimeter. when barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00 g of water in the calorimeter decreases from 30.0°c to 8.0°c. the equation for calculating energy absorbed or released by a reaction is: where q is the energy released or absorbed, m is the mass of water in the calorimeter, cp is the specific heat of water, and δt is the observed temperature change. if the specific heat of liquid water is 4.19 j/g·°c, how much energy was absorbed by the reaction?
Answers: 3

Physics, 22.06.2019 02:00, arinegrete2003
What is created when solids, liquids, an gases mix with one another
Answers: 1
You know the right answer?
A sample of quartz, which has a specific heat capacity of 0.730 Jg1, is put into a calorimeter (see...
Questions in other subjects:

Social Studies, 21.09.2021 22:40


Mathematics, 21.09.2021 22:40


Mathematics, 21.09.2021 22:40

Chemistry, 21.09.2021 22:40


English, 21.09.2021 22:40

English, 21.09.2021 22:40

History, 21.09.2021 22:40

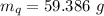
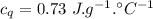
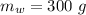
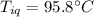
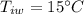
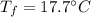
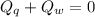
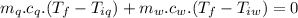
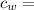 specific heat of water =
specific heat of water = 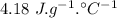 ,
,