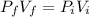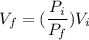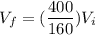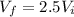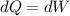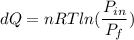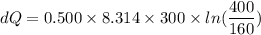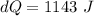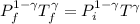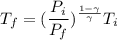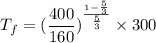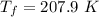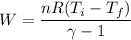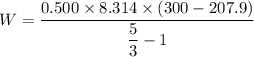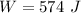Physics, 25.02.2020 04:32 rileybantaos1c8s
A 0.500-mol sample of an ideal monatomic gas at 400 kPa and 300 K, expands quasi-statically until the pressure decreases to 160 kPa. Find the final temperature and volume of the gas, the work done by the gas, and the heat absorbed by the gas if the expansion is (a) isothermal, and (b) adiabatic

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 23:00, ImBADatmath8743
Asubmarine has a "crush depth" (that is, the depth at which water pressure will crush the submarine) of 250 m. what is the approximate pressure (water plus atmospheric) at this depth? (recall that the density of seawater is 1025 kg/m3, g = 9.81 m/s2, and 1 kg/(ms2) = 1 pa = 9.8692 10-6 atm.) a. 34.8 atm b. 24.8 atm c. 25.8 atm d. 7.8 atm
Answers: 2


You know the right answer?
A 0.500-mol sample of an ideal monatomic gas at 400 kPa and 300 K, expands quasi-statically until th...
Questions in other subjects:



Mathematics, 02.05.2021 21:20

Mathematics, 02.05.2021 21:20

History, 02.05.2021 21:20