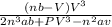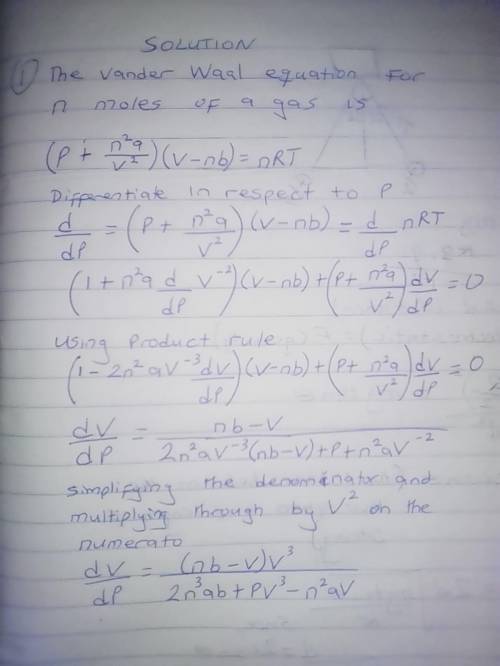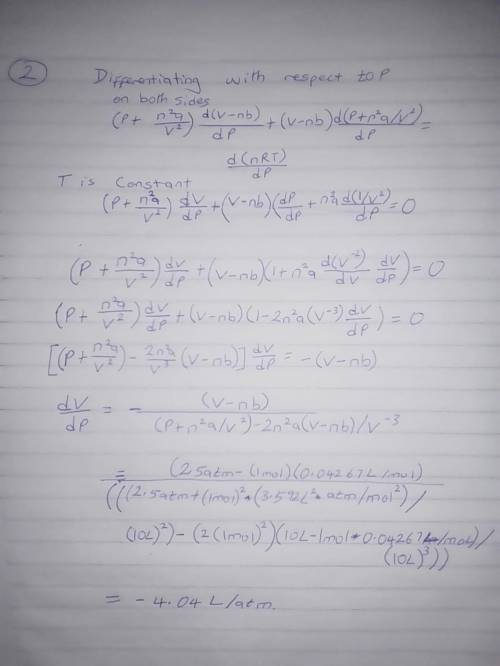Physics, 20.02.2020 16:46 izzysmith6836
The van der Waals equation for n moles of a gas is where P is the pressure, V is the volume, and T is the temperature of the gas. The constant R is the universal gas constant and a and b are positive constants that are characteristic of a particular gas. a) If T remains constant, use implicit differentiation to find . b) Find the rate of change of volume with respect to pressure of 1 mole of carbon dioxide at a volume of V = 10 L and a pressure of P = 2.5 atm. Use a = 3.592 L2 -atm/mole2 and b = 0.04267 L/mole.

Answers: 3


Other questions on the subject: Physics


Physics, 21.06.2019 23:00, xojade
Athermometer is removed from a room where the temperature is 70° f and is taken outside, where the air temperature is 10° f. after one-half minute the thermometer reads 60° f. what is the reading of the thermometer at t = 1 min? (round your answer to two decimal places.) ° f how long will it take for the thermometer to reach 30° f? (round your answer to two decimal places.)
Answers: 3

Physics, 22.06.2019 12:00, Emieloy2959
Ihave a density of 1.61g/cm^3 and a mass of 28g. find the missing value
Answers: 1
You know the right answer?
The van der Waals equation for n moles of a gas is where P is the pressure, V is the volume, and T i...
Questions in other subjects:

History, 19.04.2020 03:25



Mathematics, 19.04.2020 03:25

Mathematics, 19.04.2020 03:25




Mathematics, 19.04.2020 03:25