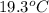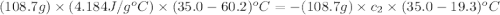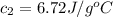A student wishes to determine the heat capacity of a coffee-cup calorimeter. After she mixes 108.7 g of water at 60.2°C with 108.7 g of water, already in the calorimeter, at 19.3°C, the final temperature of the water is 35.0°C. Calculate the heat capacity of the calorimeter in J/K. Use 4.184 J/g°C as the specific heat of water. Enter to 1 decimal place.

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 15:50, Sbudah2937
Electric charge is uniformly distributed inside a nonconducting sphere of radius 0.30 m. the electric field at a point p, which is 0.50 m from the center of the sphere, is 15,000 n/c and is directed radially outward. what is the maximum magnitude of the electric field due to this sphere
Answers: 2

Physics, 21.06.2019 20:00, berankworthy7153
Experiment scenario 2: a biology student wants to conduct a study of how the amount of food affects the weight gain for mice. i have set up four cages with a mouse in each cage. fill in the boxes that would allow the biology student to complete the study correctly
Answers: 1

Physics, 21.06.2019 22:50, ijohnh14
If the temperature were raised very high, classically what would we expect the heat capacity per object to be for this one-dimensional system? give a numerical value. chigh t = __ j/k/object (one reason for the discrepancy is that the high-temperature limit assumes that the number of oscillators is large (n > > 1), which is not the case in this tiny system.)
Answers: 2

Physics, 22.06.2019 14:30, Cnolteb5663
Slab pull” is a type of tectonic plate movement that occurs due to the forces of mantle convection and results in the subduction of the lithosphere true or false
Answers: 2
You know the right answer?
A student wishes to determine the heat capacity of a coffee-cup calorimeter. After she mixes 108.7 g...
Questions in other subjects:










Mathematics, 12.09.2019 03:30

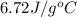
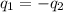
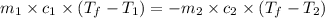
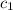 = specific heat of water =
= specific heat of water = 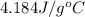
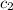 = specific heat of calorimeter = ?
= specific heat of calorimeter = ?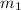 = mass of water = 108.7 g
= mass of water = 108.7 g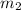 = mass of calorimeter = 108.7 g
= mass of calorimeter = 108.7 g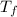 = final temperature of mixture =
= final temperature of mixture = 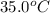
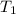 = initial temperature of water =
= initial temperature of water = 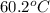
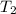 = initial temperature of calorimeter =
= initial temperature of calorimeter =