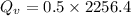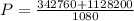Physics, 15.02.2020 03:44 aleshachrishon42
Water is boiled at sea level in a coffeemaker equipped with an immersion-type electric heating element. The coffee maker contains 1 L of water when full. Once boiling starts, it is observed that half of the water in the coffeemaker evaporates in 18 min. Determine the power rating of the electric heating element immersed in water. Also, determine how long it will take for this heater to raise the temperature of 1 L of cold water from 18°C to the boiling temperature. The enthalpy of vaporization of water at the saturation temperature of 100ºC iss hfg = 2256.4 kJ/kg. At an average temperature of (100 + 18)/2 = 59ºC, the specific heat of water is c = 4.18 kJ/kg·ºC and the density is about 1 kg/L.

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 07:20, aliviafrancois2000
Aman throws a football straight into the air. as it rises, it slows down. which type of energy is the football gaining?
Answers: 2

Physics, 22.06.2019 14:40, viktoria1198zz
14. a body is projected with velocity vi from a. at the same time another body is projectedvertically upwards from point b withvelocity v2 lies vertically below the highestpoint. both the bodies collide thenv2÷v1is
Answers: 1

Physics, 22.06.2019 17:30, janicemaxwell123
Does heating a metal wire increase or decrease its electrical resistance? why?
Answers: 1

Physics, 22.06.2019 23:30, melanyrivera776
Which model displays the change in a gas when the temperature of the gaseous system decreases?
Answers: 3
You know the right answer?
Water is boiled at sea level in a coffeemaker equipped with an immersion-type electric heating eleme...
Questions in other subjects:







Physics, 25.06.2019 18:40


Arts, 25.06.2019 18:40

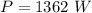
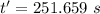 is time required to heat to boiling point form initial temperature.
is time required to heat to boiling point form initial temperature.
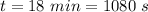
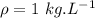

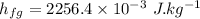
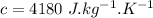
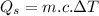
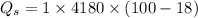
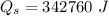

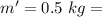 mass of water vaporized due to boiling
mass of water vaporized due to boiling