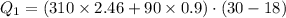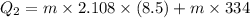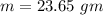Physics, 14.02.2020 19:52 chanavictor2747
Suppose 310. grams of ethanol (ethyl alcohol) is in an aluminum cup of 90.0 grams. Both of these are at 30.0C. A mass m of ice at – 8.5C is taken from a freezer and added to the alcohol in the cup. The final temperature of all the components is 18.0C. Assuming no heat was lost from the system, calculate the mass m of the ice added.

Answers: 2


Other questions on the subject: Physics

Physics, 22.06.2019 11:00, Arielledt10
1. jay fills a wagon with sand (about 20 kg) and pulls it with a rope 30 m along the beach. he holds the rope 25° above the horizontal. the rope exerts a 20-n tension force on the wagon. how much work does the rope do on the wagon?
Answers: 1

Physics, 22.06.2019 20:10, kerarucker12pe384k
Atruck with 34-in.-diameter wheels is traveling at 55 mi/h. find the angular speed of the wheels in rad/min, *hint convert miles to inches & hours to minutes:
Answers: 2

Physics, 23.06.2019 06:30, davelopez979
The energy of a photon is equal to (what) times (what) of the photon.
Answers: 1

Physics, 23.06.2019 07:00, christopherholmes
Why does an object have a different weight o the moon than it does on earth
Answers: 2
You know the right answer?
Suppose 310. grams of ethanol (ethyl alcohol) is in an aluminum cup of 90.0 grams. Both of these are...
Questions in other subjects:

Mathematics, 18.06.2020 00:57

Mathematics, 18.06.2020 00:57

English, 18.06.2020 00:57

Mathematics, 18.06.2020 00:57

Mathematics, 18.06.2020 00:57

Mathematics, 18.06.2020 00:57



Mathematics, 18.06.2020 00:57

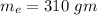
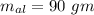
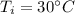
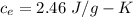
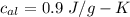
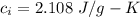
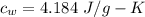
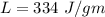
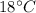 is reached
is reached