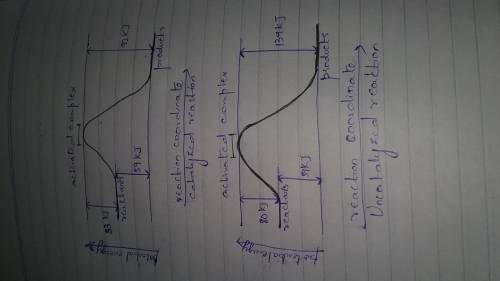
A catalyst decreases the activation energy of a particular exothermic reaction by 47 kJ/mol, to 33 kJ/mol. Assuming that the mechanism has only one step, and that the products are 59 kJ lower in energy than the reactants, sketch approximate energy-level diagrams for the catalyzed and uncatalyzed reactions.

Answers: 2


Other questions on the subject: Physics

Physics, 21.06.2019 22:00, Brandon4188
If the speed of a particle triples ,by what factor does its kinetic energy increase?
Answers: 2



Physics, 23.06.2019 00:30, gamallopatty
What is the coldest temperature ever recorded in san antonio?
Answers: 1
You know the right answer?
A catalyst decreases the activation energy of a particular exothermic reaction by 47 kJ/mol, to 33 k...
Questions in other subjects:


History, 08.05.2020 19:57



Mathematics, 08.05.2020 19:57



Mathematics, 08.05.2020 19:57