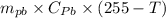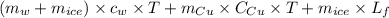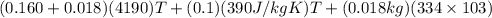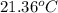A copper calorimeter can with mass 0.100kg contains 0.160kg of water and 0.018kg of ice in thermal equilibrium at atmospheric pressure.
Part A
If 0.750kg of lead at a temperature of 255 c is dropped into the calorimeter can, what is the final temperature? Assume that no heat is lost to the surroundings

Answers: 1


Other questions on the subject: Physics

Physics, 22.06.2019 09:30, gemmaestelle
Along the line connecting the two charges, at what distance from the charge q1 is the total electric field from the two charges zero? express your answer in terms of some or all of the variables s, q1, q2 and k =14ï€ïµ0. if your answer is difficult to enter, consider simplifying it, as it can be made relatively simple with some work.
Answers: 3

Physics, 22.06.2019 14:30, ayoismeisalex
Two carts, one of mass 2m and one of mass m, approach each other with the same speed, v. when the carts collide, they hook together. assume that positive momentum is to the right. which graph best represents the momentum of both carts over time, before and after the collision?
Answers: 3

Physics, 22.06.2019 17:00, bella51032
Explain what will happen if decomposers were apsent from a forest ecoysystem.
Answers: 1

Physics, 22.06.2019 19:50, aliami0306oyaj0n
State the below theories and give the name of the writer of the theory 1. wave theory 2. quantum theory 3. corpuscular theory 4. electromagnetic theory
Answers: 1
You know the right answer?
A copper calorimeter can with mass 0.100kg contains 0.160kg of water and 0.018kg of ice in thermal e...
Questions in other subjects:



Mathematics, 21.07.2019 00:40






History, 21.07.2019 00:40

History, 21.07.2019 00:40

 ) = 0.1 kg
) = 0.1 kg
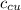 ) = 390 J/kg K
) = 390 J/kg K
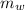 ) = 0.160 kg
) = 0.160 kg
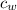 ) = 4190 J/kg K
) = 4190 J/kg K
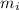 ) = 0.018 kg
) = 0.018 kg
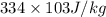
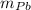 ) = 0.75 kg
) = 0.75 kg
 ) = 130 J/kg K
) = 130 J/kg K