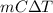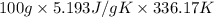Physics, 12.02.2020 02:01 loishsu4936
A mass of 0.1 kg of helium fills a 0.2 m3 rigid tank at 350 kPa. The vessel is heated until the pressure is 700 kPa. Calculate the a) the temperature change of helium [deg. C], and b) the total amount of heat required for this process [kJ].

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 21:30, galaxyworld36
Janelle made a hypothesis bout the uneven temperatures inside her house during winter. she believes that 50% of the ducts are blocked and she plans to investigate. if she wants to prove her hypothesis using scientific processes what should she do next?
Answers: 2

Physics, 22.06.2019 10:10, randlemccray4305
What is the angular momentum of the bar about the axle?
Answers: 3

Physics, 22.06.2019 10:30, elijahjacksonrp6z2o7
The freezing and boiling point of a substance changes as the air pressure around it changes. for example, at a lower air pressure (higher altitude) it is easier for water molecules to escape from liquid into the air. in a high altitude city such as denver, colorado compared to a sea-level city such as houston, texas, water
Answers: 2

Physics, 22.06.2019 15:50, rosepetals2938
If the work required to stretch a spring 3 ft beyond its natural length is 15 ft-lb, how much work is needed to stretch it 27 in. beyond its natural length?
Answers: 1
You know the right answer?
A mass of 0.1 kg of helium fills a 0.2 m3 rigid tank at 350 kPa. The vessel is heated until the pres...
Questions in other subjects:



Mathematics, 23.07.2019 11:20



Biology, 23.07.2019 11:20



English, 23.07.2019 11:20

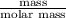
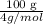
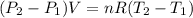
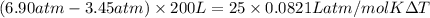
 = 336.17 K
= 336.17 K