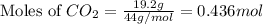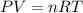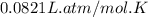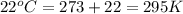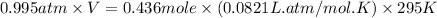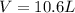Physics, 11.02.2020 23:31 kiarabermudez754
A 19.2g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus. Calculate the expansion work done against a constant external pressure of 0.995 atm and at a constant temperature of 22 degrees C. Assume that the initial volume of dry ice is negligible and that CO2 behaves like an ideal gas.

Answers: 2


Other questions on the subject: Physics



Physics, 22.06.2019 18:50, rurbanok12
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3

Physics, 22.06.2019 22:00, tewilliams1
If anyonee has done the momentum lab practical in physics can you give me the answers i am s lostt rnn : (
Answers: 2
You know the right answer?
A 19.2g quantity of dry ice (solid carbon dioxide) is allowed to sublime (evaporate) in an apparatus...
Questions in other subjects:


Mathematics, 01.09.2021 04:00

Physics, 01.09.2021 04:00

Chemistry, 01.09.2021 04:00

Mathematics, 01.09.2021 04:00





Social Studies, 01.09.2021 04:00

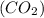 .
.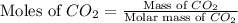
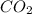 = 44 g/mole
= 44 g/mole