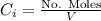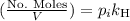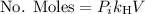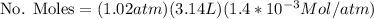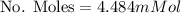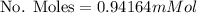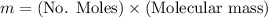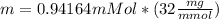Physics, 28.01.2020 20:48 Rodrigo6379
Calculate the mass of oxygen (in mg) dissolved in a 3.14 l bucket of water exposed to a pressure of 1.02 atm of air. assume the mole fraction of oxygen in air to be 0.21 and the henry's law constant for oxygen in water at this temperature to be 1.4 × 10-3 m/atm o2. (enter your value using three significant figures.)

Answers: 3


Other questions on the subject: Physics

Physics, 22.06.2019 05:10, delawdermia27
Which diagram correctly demonstrates the various forces acting on a ball moving horizontally with some speed?
Answers: 2

Physics, 22.06.2019 14:30, willoughbysierra
The man of mass m1 and the woman of mass m2 are standing on opposite ends of the platform of mass m0 which moves with negligible friction and is initially at rest with s = 0. the man and woman begin to approach each other. derive an expression for the displacement s of the platform when the two meet in terms of the displacement x1 of the man relative to the platform.
Answers: 1

Physics, 22.06.2019 15:30, jjjjjj4999
Match each scenario to the form of energy it represents
Answers: 2

Physics, 22.06.2019 22:00, kelsea86
The pilot of a helicopter hovers at an altitude of 1200 feet over a park. the angle of depression to the base of a statue is 17 degrees. the angle of depression to the nearest park exit, in line with the statue, is 14 degrees. to the nearest foot, what is the distance from the statue to the exit?
Answers: 1
You know the right answer?
Calculate the mass of oxygen (in mg) dissolved in a 3.14 l bucket of water exposed to a pressure of...
Questions in other subjects:



Mathematics, 03.06.2021 18:10

Mathematics, 03.06.2021 18:10



Mathematics, 03.06.2021 18:10

Social Studies, 03.06.2021 18:10


Mathematics, 03.06.2021 18:10

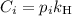
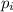 is the partial pressure of the gas.
is the partial pressure of the gas. is the concentration of the gas (solubility).
is the concentration of the gas (solubility).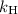 is Henry's constant, which depends on the nature of the gas, the temperature and the liquid.
is Henry's constant, which depends on the nature of the gas, the temperature and the liquid.