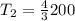Physics, 27.01.2020 22:31 letsgetcookingblog
Suppose that 10 moles of an ideal gas have a gauge pressure of 2 atm and a temperature of 200 k. if the volume of the gas is doubled and the pressure dropped to a gauge pressure of 1 atm, what is the new temperature?
select one:
a.
267 k
b.
300 k
c.
400 k
d.
200 k

Answers: 1


Other questions on the subject: Physics

Physics, 21.06.2019 17:00, pinkfluffyunicorns
These models show the electron structures of two different nonmetal elements. which element is likely more reactive, and why? element 1 is more reactive because it has fewer electron shells and is toward the top of its group on the periodic table. element 1 is more reactive because it has more electrons in its valence shell and is farther to the right on the periodic table. element 2 is more reactive because it does not have a valence shell close to the nucleus, so it will attract electrons. element 2 is more reactive because it does not have a full valence shell, so it will attract electrons.
Answers: 2

Physics, 22.06.2019 02:00, arinegrete2003
What is created when solids, liquids, an gases mix with one another
Answers: 1

Physics, 22.06.2019 05:30, dxpebetty64
Astudent pushes on a 20.0 kg box with a force of 50 n at an angle of 30° below the horizontal. the box accelerates at a rate of 0.5 m/s2 across a horizontal floor. what is the value of the normal force on the box? 200 n 243 n 156 n 225 n
Answers: 2
You know the right answer?
Suppose that 10 moles of an ideal gas have a gauge pressure of 2 atm and a temperature of 200 k. if...
Questions in other subjects:

English, 20.01.2020 09:31

History, 20.01.2020 09:31

Mathematics, 20.01.2020 09:31




Geography, 20.01.2020 09:31

History, 20.01.2020 09:31

Mathematics, 20.01.2020 09:31