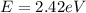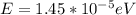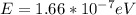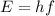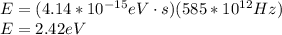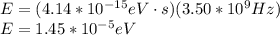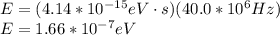Calculate the energy, in electron volts, of a photon whose frequency is the following.
...

Calculate the energy, in electron volts, of a photon whose frequency is the following.
(a) 585 thz
(b) 3.50 ghz
(c) 40.0 mhz
planck's equation for the energy of a photon is e = hf, where f is the frequency and h is planck's constant. we use 1 ev = 1.60 ✕ 10−19 j for units of energy.
(a) for the energy of the photon at a frequency of 585 thz, we have e = hf

Answers: 3


Other questions on the subject: Physics

Physics, 21.06.2019 17:00, pinkfluffyunicorns
These models show the electron structures of two different nonmetal elements. which element is likely more reactive, and why? element 1 is more reactive because it has fewer electron shells and is toward the top of its group on the periodic table. element 1 is more reactive because it has more electrons in its valence shell and is farther to the right on the periodic table. element 2 is more reactive because it does not have a valence shell close to the nucleus, so it will attract electrons. element 2 is more reactive because it does not have a full valence shell, so it will attract electrons.
Answers: 2


Physics, 22.06.2019 00:30, adantrujillo1234
What are the theoretical properties of a gas at a temperature of 0 kelvin?
Answers: 3

Physics, 22.06.2019 10:00, ceasar6071
Students design a model roller-coaster track. they place a rubber ball at the highest point on the track and let it go. the ball rolls along the track pulled only by the force of gravity. eventually, it comes to a stop. which change to the design will result in the ball moving the greatest distance?
Answers: 1
You know the right answer?
Questions in other subjects:


Spanish, 28.06.2019 10:00








Spanish, 28.06.2019 10:00